Discover the Best 5 Strategies for Understanding Keto-Enol Tautomerism in 2025
Introduction to Keto-Enol Tautomerism
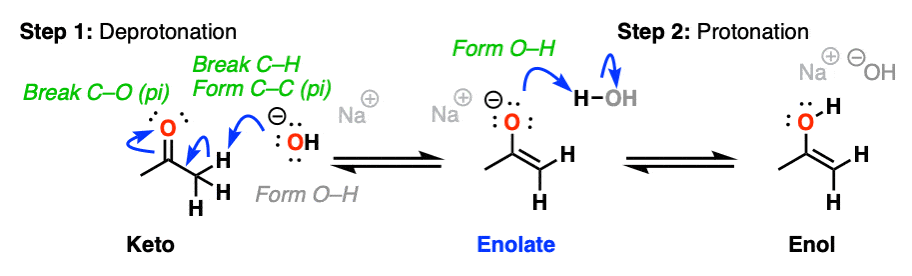
Keto-enol tautomerism is a fascinating and complex area of organic chemistry, involving the interconversion between two isomers: the **keto form** and the **enol form**. This dynamic equilibrium between tautomers plays a significant role in various chemical reactions, emphasizing the underlying importance of **reaction mechanism** and **molecular structure**. In this article, we will explore five essential strategies to enhance your understanding of this subject, particularly focusing on **tautomerization**, **chemical equilibrium**, and practical applications. By grasping these concepts, you’ll gain insights into how molecular dynamics and environmental factors influence keto-enol behaviors and their applications in real-world chemistry.
1. Mastering the Basics: Understanding Tautomerization
To comprehend keto-enol tautomerism, begin with the foundational concepts of **tautomerization**. Tautomerization refers to the rapid conversion between isomeric forms due to the transfer of hydrogens, exemplified by the keto-enol pair. In most cases, the **keto form** is more stable than the **enol form** under standard conditions; however, environmental factors such as solvent effects and temperature can significantly impact this equilibrium. Recognizing how **hydrogen bonding** and **proton transfer** relate to the structural preferences of these forms will set you up for advanced study. Additionally, familiarizing yourself with **tautomeric shifts** can help delineate scenarios where enols can predominate, an essential insight especially in biochemical reactions.
2. Exploring Reaction Mechanisms in Detail
Diving deeper into the **reaction mechanism** of keto-enol tautomerism illuminates how reactions unfold at the molecular level. Key elements include recognizing **bond lengths**, **bond angles**, and the role of **stereoisomerism** in determining the stability and accessibility of different tautomers. The **reaction pathway** for keto-enol conversion can also involve **electrophilic addition**, depending on the specific functional groups present in the compounds. Utilizing resources like **NMR analysis** and **UV-Vis spectroscopy** can aid in identifying tautomeric forms and measuring **keto-enol ratios**, supplying essential data for theoretical and experimental studies. Understanding the energetics outlined in an **energy diagram** enhances comprehension of the underlying forces at play in dependency on chemical conditions.
3. Investigating Thermodynamic and Kinetic Control
An essential aspect of keto-enol tautomerism is differentiating between **kinetic versus thermodynamic control**. The stability of the keto and enol forms depends not only on the energy landscape but on reaction conditions such as pH and temperature. It’s crucial to examine how the **equilibrium constant** is affected by various solvents and external conditions. This understanding aids in predicting the outcomes of reactions involving **1,3-dicarbonyl compounds** like **acetylacetone**, known for their ability to form stable enolic forms. Developing insights into **reaction kinetics** by studying rate constants can also facilitate clearer predictions of products in synthetic applications. Analytical techniques such as **computational modeling** and **density functional theory** play a vital role in elucidating these factors comprehensively.
4. Practical Applications in Chemistry
The applications of keto-enol tautomerism extend widely into fields like drug design and materials science. By leveraging the principles of **tautomeric equilibria**, chemists can design molecules with desired properties, taking into account **intermolecular interactions** and molecular dynamics. In biologically relevant systems, understanding **tautomerization in natural products** illustrates how enolate formation can influence reactions. Likewise, reactions involving **tautomerization** can yield new pathways for synthesizing complex compounds. Familiarity with these methods not only enriches theoretical knowledge but facilitates practical laboratory experiments showcasing tautomerism effects, particularly for students and researchers involved in chemical synthesis.
5. Embracing Modern Techniques for Analysis
Lastly, integrating **spectroscopy techniques** and computational predictions can significantly enhance your grasp of keto-enol tautomerism. Employing methods such as **molecular orbital theory applications** supports deeper insights into the stabilization of tautomers through resonance structures. Additionally, **kinetic isotopic effects** may provide illustrative examples of how isotopic composition influences tautomeric ratios, allowing for rigorous studies in organic chemistry. Embracing modern technological approaches not only streamlines data acquisition but also bolsters experimental learning through real-life applications in various chemical contexts. Practical experiments in tautomerism can reinforce these concepts, showcasing their relevance in chemical interactions.
Key Takeaways
- Understand the basics of keto-enol tautomerism through the dynamics of molecular structures and tautomerization.
- Delve into the reaction mechanisms to observe the relationship between kinetic and thermodynamic factors.
- Consider practical applications in drug design and molecular synthesis to illustrate the theory in action.
- Utilize modern spectroscopy and computational techniques for profound insights into tautomeric behavior.
FAQ
1. What are the differences between keto and enol forms?
The **keto form** typically features a carbonyl group (C=O) attached to two carbon atoms, whereas the **enol form** contains an alkene with an alcohol (C=C-OH) configuration. This structural difference imparts varying degrees of stability between these tautomeric forms, manifesting across diverse solvents and reaction conditions.
2. How does solvent choice influence keto-enol tautomerism?
Solvents can greatly affect the **keto-enol equilibrium** by stabilizing one tautomer over the other, influenced by factors like dielectric constant and intermolecular interactions. Protic solvents may stabilize the enol form through hydrogen bonding, thereby modulating the **keto-enol ratios** in a reaction.
3. What role does temperature play in tautomerization?
Temperature significantly impacts the **reaction kinetics of keto-enol tautomerism**. Higher temperatures can facilitate a faster tautomerization rate by increasing molecular vibration and consequently lowering the energy barriers that separate the tautomers, thereby favoring the more thermodynamically stable form.
4. How can spectroscopy be utilized in studying tautomerism?
Spectroscopy techniques like **NMR analysis** and **UV-Vis spectroscopy** are crucial for identifying different tautomers and elucidating their ratios. By tracking shifts in chemical shifts or absorbance, researchers can effectively ascertain the presence and concentration of **tautomeric forms** in various chemical environments.
5. Can tautomerism impact drug design?
Yes, understanding **tautomerism in drug design** is essential, as the activity of pharmaceutical agents can be influenced by their tautomeric forms. Proper consideration of possible keto-enol conversions enables chemists to optimize compounds for better bioavailability and efficacy.